1.0
Purpose
1.1 To evaluate, control and document the
deviations (and batch deviations, reprocessing for approval or rejection) to
ensure that product quality, safety, purity and efficacy is maintained and the activities are in accordance
with the frame work of cGMP. And It also ensures that the investigation (if required) is carried out,
recorded and any cause or causes identified and necessary corrective and
preventive measures are taken. If required to bring the deviation under the
notice of the concerned management of NAME Pharmaceuticals Ltd.
2.0
Scope
2.1 This SOP is applicable for all
products, chemicals & packaging materials, the processes, systems, equipment’s,
instrumentation, utilities and facilities used to produce and control them and
be followed by all departments of NAME Pharmaceuticals Ltd.
3.0
Responsibility
3.1 Head of Quality Assurance or His / Her
Nominee:
1. Review
deviations in relation to their impact on quality and GMP compliance.
2. Ensure
this procedure covers the initial identification, assessment, investigation (if
required), report preparation and remedial action proposal to prevent
recurrence.
3. Ensuring
that the system for investigation of failure and the effectiveness of
corrective actions is managed and monitored.
4. Review
of any Environmental, Health or Safety implications from the deviation.
5. Implement
decision regarding remedial action through change control (if required), if
required Regulatory for those
batch deviations having potential regulatory impact.
6.
Ensure the documentation of all deviation
records for the trend analysis of deviations.
3.2 Originator:
1. Ensuring
that the details, including any immediate action taken, of the occurrence of a
deviation are recorded at the time of the incident and any further action is
agreed with the Departmental Manager.
3.3 Departmental Manager / Head / In-Charge
1. Ensuring
that Head of Quality Assurance or His / Her Nominee are informed promptly and
that in conjunction with any / or other relevant function, assessing whether
there is impact on product quality and / or regulatory compliance. Ensuring
that any immediate actions taken are appropriate, discussing with the above and
the details of the occurrence has been reported.
2. Authorizing
any Corrective Actions and ensuring any Preventative Actions are identified and
completed against an agreed time scale.
3. Ensuring
that the deviation is logged, monitored and assessed and summaries provided.
3.4 Investigation Team
1. An investigation team should be constituted
comprising members from Quality Assurance / Control and Other Department(s)
depending on the nature of the deviation.
2. Ensuring
that an investigation is carried out, this will establish the cause (root
cause), scope and impact of the deviation.
3. Ensuring
that the investigation team comprises relevant experts.
4. Ensuring
that all aspects of investigation are carried out and reported to an agreed
time scale
5.
Ensuring that any actions required to prevent
recurrence are identified.
4.0 Abbreviations and Definitions
4.1 Deviation:
Any planned or unplanned event or failure from Standard Operating Procedure and or failure to meet specified limits. This may include, but is not limited to:
Ø Alert
/ Action Limits exceeded.
Ø Instrument
outside of its calibration date and / or tolerance.
Ø Environmental
results outside of specification.
Ø Deviation
from SOP or processing limits in manufacture or testing.
Ø Process,
equipment, procedure or system failure.
Ø Material
accountability discrepancy.
Ø Damage
in storage or handling.
Ø Out of
Specification result.
Ø Component
/ supplier problem
Ø Operator
errors.
Deviation must be recorded, investigated (if required), reported, and included in any monitoring / trending information; note that, product rejection may or may not result. The deviation can be classified as follow.
4.1.1. Critical Deviation
If the unplanned deviation that has anticipated
a serious impact on product quality which could cause either adverse health
consequence or regulatory impact and needs urgent attention of all departments
concerned, will investigate through investigation committee, prior to any
decision about release or reject.
4.1.2. Major
Deviation
The unplanned deviations that having an impact
on product quality and therefore need to be thoroughly evaluated and
investigated prior to any decision about release or reject through
investigation committee.
4.1.3. Minor Deviation
Minor deviations are those which are unplanned deviation, are not critical to product quality, need to be documented, reviewed and approved.
4.2 Fail / Reject
The status of a product or material, which has either directly or indirectly been the subject of a deviation / failure investigation, and the scientifically justified outcome of the investigation is that the product / material does not meet either its Quality specification(s), or comply with registered information.
4.3 Investigation
The systematic process by which the deviation and supporting evidence is examined to determine causal factors, the root cause, the impact on product / batch / material quality, established, corrective actions required determined, and a documented report generated.
4.4 Immediate Action
Actions taken at the time of an incident to make the process / product / system / material safe and secure, and / or prevent further deterioration.
4.5 Corrective Action
Remedial action taken at the time of the event or identified as part of the (root cause) investigation, that is necessary to recover the product / process / material / system, affected by the deviation.
4.6 Preventative Action
Actions identified as part of the (root cause) investigation, which must be carried out to prevent the Non-conformance recurring. Preventative actions may or may not be completed prior to a decision on batch disposition.
4.7 Reprocessing
The treatment of all or a part of a batch of
product of an unacceptable quality from a defined stage of production with the
original process so that its quality may be rendered acceptable by one or more
additional operations.
5.0 Materials and Equipment
5.1 None
6.0 Precaution / Health and Safety Considerations
6.1 None
7.0
Procedure
7.1 Numbering and Logging of Deviation
7.1.1 Record the deviation in details in the Deviation Logbook (Annexure-II) and update the records and maintain as the investigation proceeds. Details to be completed in the log include:
Ø Date
of occurrence
Ø Reference
number - Unique reference number (described in the following section 7.1.2)
Ø Product
/ Material / Equipment name
Ø Batch
/ Code / ID number of the corresponding above name
Ø A
single sentence summary statement of the details of the deviation
Ø Investigation
close out date (investigation is required or not) - Agreed time scale for
corrective
actions or investigation close out date.
Ø Further
investigation closed date – Date of confirmation of completion of corrective
actions
or further investigation closed date.
Ø Where
or not product failure is implicated.
Ø Close
out date of preventive actions - Agreed time scale for preventive actions
Ø Preventive
Actions closed date - Date of confirmation of completion of preventative action
Ø Final
close out date.
State N/A where any column is not required to fulfil.
7.1.2 Follow the unique reference number format as following:
D/DC/ XXX/YY
D = Deviation
DC = Department
Code
XXX = Three-digit
sequential number, commencing 001, taken as the next sequential number
from the logbook
YY = Last two digits of the current year.
Deviation Department
Codes (DC):
|
Department
Name |
Abbreviated
Department Code |
|
Quality
Assurance |
QA |
|
Quality
Control |
QC |
|
Microbiology |
MB |
|
Production |
PR |
|
Engineering |
EN |
|
Warehouse |
WH |
|
Product
Development |
PD |
7.2 Deviation Identification, Action Taken and Notification
7.2.1 When any deviation /incidents come to the notice of any personnel, the observer will immediately stop the operations and notify the incident to Head of concerned department, who discuss the matter with Head of Quality Assurance or His / Her Nominee.
7.2.2 Collect data / information pertaining to the deviation, and review the Departmental Manager, who will confirm the occurrence of the deviation.
7.2.3 Notify Head of Quality Assurance or His / Her Nominee regarding the deviation. Discuss the type of the deviation considering the impact on the product quality.
7.2.4 On agreement of the deviation, raise a Deviation Management Form (Annexure-III) with the next sequential unique reference number taken from the deviation logbook (Annexure-II)
7.2.5 Complete the Deviation Management form (Annexure-III).
7.2.6 Review and assess the type of the deviation weather the deviation is critical or major or minor type.
7.2.7 Minor deviations are needed to be documented, reviewed and approved by the Head of Quality Assurance or His / Her Nominee.
7.2.8 Investigate the major and critical type deviation prior to any decision about release. But if Head of Quality Assurance or His / Her Nominee decides to investigate the issue then the deviation will be investigated prior to any decision about release.
7.2.9 During investigation find out the root cause and also identify corrective and preventive actions necessary. If the root cause could not be identified then the investigation committee will decide the further action to be taken.
7.2.10 Take decision regarding the fate of the batch such as approval or rejection. If approved, take decision that additional testing, stability evaluation or validation or reprocessing is required or not.
7.2.11 Write the procedure for reprocessing of batches that do not conform to standards or specification. Get approval of the procedure of reprocessing from Head of Quality Assurance or His / Her Nominee and also follow change control procedure if the reprocessing steps are being permanent for the future.
7.2.12 Reprocessing procedure must define steps to be taken to ensure that the re-processed batches will conform with all established standards, specifications and characteristics and assess regarding process validation and stability implications.
7.2.13 Reprocessing of product is exceptional and must not be done on a routine basis.
7.2.14 Decide whether additional testing, stability evaluation of reprocessed batch is required or not and validation of the product is required or not.
7.2.15 Special consideration should be given for the need to revalidation.
7.2.16 Archive all records of reprocessing.
7.2.17 Raise change control if permanent change is required.
7.2.18 Keep the product of deviation and or for reprocessing in a separate area.
7.2.19 Evaluate and close out the deviation in a timely manner (normally within 30 calendar days).
7.2.20 Consider that the incomplete step (continuation of a process after an in-process control test) as a part of the normal process, not as reprocessing.
7.2.21 Document supplemental filing to allow for (planned) Reprocessing in the course of commercial Manufacturing, take approval by the Regulatory authority.
7.2.22 Give special consideration for the need to revalidate and for the need to perform additional stability studies.
7.3 Conduct Investigation
7.3.1 Appoint an investigation leader and the team to carry out investigation by Head of Quality Assurance or His / Her Nominee.
7.3.2 Complete the investigation in a timely manner. Carry out investigation on the basis / type of the deviation.
7.3.3 Generate a report on completion of the investigation, summarize the outcome, with respect to effect on quality, root cause of the incident, corrective and preventative actions.
7.3.4 The investigation must include the evaluation and suitability of the affected batches for market/clinical use.
7.3.5 Complete investigations of the deviation within 30 (thirty) working days.
NOTE:
Ø Head
of Quality Assurance or His / Her Nominee must be notified of all
Investigations that are open beyond 10 (ten) working days.
Ø Investigations
that exceed 10 (ten) working days must be justified and include action plans
with time commitments.
7.4 Closure, Archiving and Trend Analysis
7.4.1 Detect the recurrent problems.
7.4.2 Implement corrective action or preventative action, if any.
7.4.3 Update the deviation logbook.
7.4.4 Sign the section 8 of Annexure-III with decision and remark to close the deviation report.
7.4.5 Send the completed deviation report and accompanying documents (if any) to Officer /Sr. Officer QA for archival at the deviation file.
7.4.6 Send the photocopy of the completed deviation report to the Department from where the deviation is raised and also attach a copy with the relevant batch manufacturing record.
7.4.7 Include a summary of all deviation in the annual report for trend analysis.
NOTE:
Ø In
the event of Out of Specification (OOS) or A typical laboratory results the investigation
must follow the relevant SOP - Evaluation of Analytical Test Result and
Investigation of Out Of Specification Test Result (OOS)
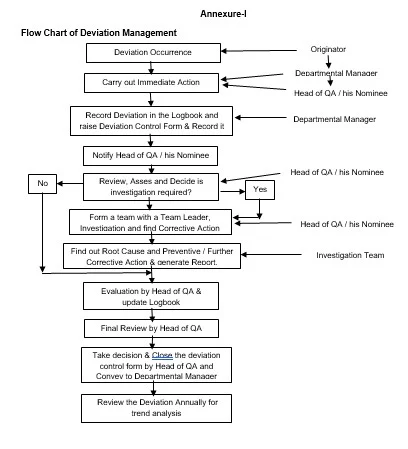
Ø The
procedure is illustrated as a flow chart in Annexure-I.
7.5 Content of Deviation Management Form (Annexure-III)
7.5.1 Section 1:
Originating Department - Name of the
originating department raising deviation form
Date – Date of raising deviation form
Date of Incident - Date of deviation
occurrence
Incident Batch Linked - Yes / No (tick
which is appropriate)
Batches Affected – Batch Nos. of the
products that are affected.
7.5.2
Section
2:
Product
Name - Name the product for which deviation is raised
Batch
Number - Deviation is raised for batch number of the product
Date of
Manufacturing - Date of manufacturing of the mentioned product / material
Date of Expiry - Date of expiry of the
mentioned product / material
Supplied
Material - Name the supplied material for which deviation is raised
Supplier
Lot Number - Deviation is raised for lot number of the supplied material
Quantity -
Quantity of the batch affected / supplied material
Process –
Name of the process for which deviation is raised
Machine -
Name of the machine for which deviation is raised
System - Name of the system for which
deviation is raised
7.5.3
Section
3:
Deviation (detailed description of the deviation, if
required use extra page) - Originator will write up the incident of deviation
and also write up his name, signature and date.
7.5.4
Section
4:
Immediate
Corrective Action – Mention the decision that was taken an immediate corrective
action by originator and departmental manager in consultation with Head of
Quality Assurance or His / Her Nominee to make the process / product / system /
material secure, to prevent further deterioration. Departmental Manager will
write up the Immediate Corrective Action with signature and collect signature
from the above person mentioned.
7.5.5
Section
5:
Investigation
(if required) and Corrective Action (attach any additional information on a
separate sheet) - Investigation Team Leader will write up the Investigation
Report and Corrective Action with signature and collect signature from Head of
Quality Assurance or His / Her Nominee.
7.5.6
Section
6:
Possible
Root Cause and Preventive Action - Investigation Team Leader will write up the
Possible Root Cause and Preventive Action in consultation with Head of Quality
Assurance or His / Her Nominee with signature. Mention the preventive Action
with the person responsible for and the completion date.
7.5.7
Section
7:
Evaluation
(Product Impact) - In Head of Quality Assurance or His / Her Nominee will write
down the impact of this deviation on the product (considering the investigation
report) and corrective action, root cause and preventive action with signature
and date.
7.5.8
Section
8:
Closure
- In closure the Departmental Manager in consultation with Head of Quality
Assurance or His / Her Nominee will decide to accept or reprocess or require
additional IPQA / QC test or require further validation / revalidation or
stability test or reject with signature. Mention the completion date. Other
than the above write it down at the remarks.
8.0
Reference
Document
8.1 In-house
9.0
Annexure
9.1 Annexure-I : Flow Chart of Deviation Management
9.2 Annexure-II : Format of “Logbook for Deviation Management”
9.3
Annexure-III : Deviation Management Form
10.0 Revision History
Revision No. | Brief reason for the revision | Effective Date | Remarks |
01 | New |
11.0
Training
11.1 Head of Quality Assurance or his nominee
give the training of production operator and worker.
Annexure-III
Deviation
Control Form
Section 1 Section 2
|
Originating
Department: |
Product
Name: |
|
Batch No.: |
|
|
Date: |
Date of
Mfg.:
Date of Exp.: |
|
Supplied
Material: |
|
|
Date of Incident: |
Supplier
Lot No.: |
|
Quantity: |
|
|
Incident
Batch Linked: Š Yes / ŠNo |
Process:
|
|
Batches
Affected: |
Machine: |
|
System: |
Section 3
|
Deviation (detailed
description of the deviation, if required use extra page)
Originator’s Name:
Signature: Date: |
Section 4
|
Immediate Corrective Action
(Departmental Manager) (Sr. /Officer QA ) / (Head of QA) |
Section 5
|
Investigation
(if required) & Corrective Action (attach any additional information on a
separate sheet)
(Investigation Team Leader) (Sr. /Officer QA ) / (Head of QA) |
Section 6
|
Possible
Root Cause
Preventive Action |
||
|
Action |
Responsibility |
Completion Date |
|
|
|
|
|
(Investigation Team Leader) (Sr. /Officer QA ) / (Head of QA) |
||
Section 7
|
Evaluation
– Product Impact
(Sr. /Officer QA ) / (Head of QA) |
Section 8
|
Closure (Tick the
following): |
|||||
|
Accept |
Reprocess |
Additional
QA/QC
Test |
Further
Validation/ Revalidation |
Stability
Test |
Reject |
|
Completion
Date: Remarks
(Departmental Manager) (Sr. /Officer QA ) / (Head of QA) |
|||||